
Thermal Properties and Temperature
Thermal Expansion of a Solid
When solids are heated, they expanded by a small amount (usually too small to see with the naked eye).
This expansion occurs because the particles within the solid gain more kinetic energy as the temperature goes up, and therefore vibrate more vigorously and gain more separation from neighboring particles.
In structures like railway tracks, the thermal expansion of the track can cause misalignment problems.
Therefore, railway tracks are made with small gaps between each rail in order to account for the slight expansion on a hot day. If this was not done then they would be forced against each other and the track would bend.
Thermal Expansion of a Liquid
Liquids expand more than solids when heated, and is easily visible. Simple liquid-in-glass thermometer use this concept.
When the thermometer is placed in a hot liquid, the alcohol (or mercury) inside the thermometer expands. This forces the alcohol up the narrow tube so that the thermometer gives a higher temperature reading.

Thermal Expansion of a Gas
Gases expand more than liquids when heated. When temperature is increased, the particles gain more kinetic energy and thus move a lot faster. This means that they take up more space and if the gas is free to expand, then it will.
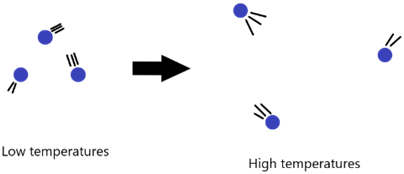
Measurement of Temperature
Physical properties that vary with temperature – Physical properties that vary with temperature are used for the measure of temperature.
For example:
- Thermal expansion (used in liquid-in-glass thermometers)
- Electrical resistance (used in thermistors)
- Potential difference (used in thermocouples)
Fixed points – To define a temperature scale, two reference temperatures called fixed points must be chosen. This fixed scale is important to universalize the temperature measurements, so that we are always comparing tomatoes with tomatoes, rather than say, oranges.
These are temperatures at which certain particular physical properties manifest themselves i.e. melting, boiling
- The Celsius scale is defined by the freezing point of water (0) and boiling point of water (100)
Sensitivity, Range, and Linearity – Consider a simple liquid-in-glass thermometer. Temperature is measured via the expansion of liquid with increased temperatures.
However, that is not to say that the liquid expands exactly the same amount for each temperature rise. In other words, the liquid expansion with temperature is not perfectly linear.
The thermometer must there be calibrated to account for this non-linearity. The sensitivity of the thermometer describes how accurately it was calibrated.
- Liquid in glass thermometers generally achieve measures to the nearest 1 °C
- Digital thermometers can achieve to the nearest 0.1°C
A liquid-in-glass thermometer cannot measure temperatures below the freezing point of water, and above the boiling point of the liquid being used. This is called the range.
Types of thermometers
Liquid-in-glass thermometers – As discussed above, these thermometers use liquid expansion to measure temperature.
The advantages of these thermometers are that they are easy to use and convenient to carry around.
The downside however is that they have limited range (i.e. 0-100) and cannot measure rapidly changing temperatures due to the fact that it takes a relatively long time for the liquid to expand and give us a reading.
Thermocouple – A thermocouple is a type of thermometer that uses voltage differences in order to measure temperature.
This digital equipment has the advantage of being able to measure a much larger range of temperatures, and more accurately, than the liquid-in-glass.
Moreover, because they give instant temperature readings, they can provide accurate measurements even if the temperature of the substance is changing rapidly.
Thermal capacity (heat capacity)
Internal energy – When an object is heated, the particles within the object gain more kinetic energy as they moved faster. This means the object’s internal energy increases.
Thermal capacity – Thermal capacity, also referred to as heat capacity, is the amount of energy required to change the temperature of an object by a certain degree. The thermal capacity of an object is dependent on the material from which it is made, and the mass.
Specific heat capacity – Specific heat capacity, also referred to as the specific heat of a material, is the amount of energy needed to raise the temperature of an object per unit mass of that object. In other words, it is the energy required to raise the temperature of a 1kg material by 1°C. For example, the specific heat capacity of water is 4200 J (Kg°C), meaning it takes 4200 J of energy to raise the temperature of 1Kg of water by 1°C.
Energy = Mass x Specific Heat Capacity x Temp. Change.
E = mcΔT
Experiment to determine specific heat capacity of water
Background information – In order to find the specific heat capacity of any substance, we must re-arrange the equation as follows:
E = mcΔT
We therefore need to find out the following:
- Mass of substance (m)
- Temperature change of substance (ΔT)
- Energy used to cause this temperature change (E)
Experimental arrangement –
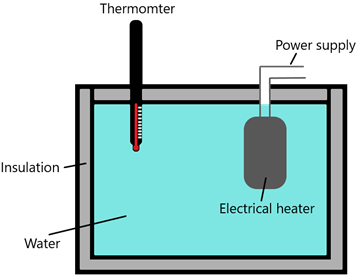
Procedure –
- 0.50 kg water is used into a container with insulation
- A thermometer is used to measure the temperature of the water
- An electrical heater with known power (50 W) is placed in the water
- Initial temperature reading is taken
- The heater is switched on and a timer is started simultaneously
- Timer is stopped when the temperature rises by 10°C
In this example, just say a 50W heater took 7 minutes (420s) to heat water from 40°C to 50°C
Calculations –
- Energy supplied by heater (E) = power X time = 50W X 420s = 21 000J
- Mass of water (m) = 0.50 kg
- Change in temperature (ΔT) = 10°C
Applying the formula –
c = 4200 J/(Kg°C)
In reality a lot of energy from the heater would not be transferred 100% to the water, so the value would be a bit different from 4200.
Melting and Boiling
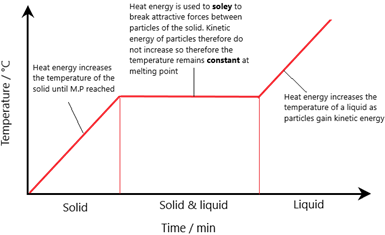
Melting – Change in state of a solid to a liquid. The temperature at which a solid, turns into a liquid is called the melting point.
Imagine heating a solid:
- As it is heated, the temperature increases until the melting point is reached
- Once the melting point is hit, the solid will start to become a liquid. During this transition phase, despite the energy (heat) input, the temperature remains constant.
Once the solid has fully melted into a liquid, the heat finally begins to raise the temperature of the liquid
The key point is that during the transition phase from a solid to liquid, the heat does not increase the temperature.
Condensation is the reverse of boiling. Gas particles can lose energy resulting in low energy positions. During the transition from a gas to liquid, the kinetic energy of particles remains unchanged and therefore the temperature remains constant (just like from a liquid to gas). The energy which must be added to vaporize a liquid at boiling point, or given out when a gas condenses, is called the latent heat of vaporization.
Boiling vs evaporation – Both boiling and evaporation exemplify a state change from a liquid to gas. However, it is extremely important to understand the crucial differences between these two terms:
- Boiling occurs at a fixed temperature which depends on the substance being heated and the pressure
- Evaporation can occur at all temperatures, including below the boiling point
- Evaporation decreases the temperature of the remaining liquid. In boiling however, the temperature remains constant.
Specific Latent Heat
Important formula
Energy Transferred = Δ Mass x Specific Latent Heat
E = Δ mL
Specific latent heat of fusion – Energy required to melt 1 kg of solid at its melting point, with no change in temperature. For example, the specific latent heat of fusion of water is 300, 000 J/Kg. This means that it takes 300, 000 J of energy to melt 1kg of pure ice at 0°C
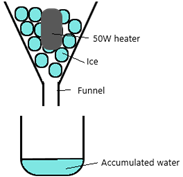
The specific latent heat of ice can be calculated using this procedure:
- Fill a funnel with ice and place a beaker beneath it
- Place a 50W heater in the ice
- Turn on the heater & start the timer immediately
- After 10 minutes turn off the heater
- Measure the mass of the accumulated water in the beaker
Assume that we accumulated a total of 0.1L of water in 10 minutes (600 seconds)
Calculations:
- Energy supplied (E) = power X time = 50 X 600 = 30 000J
- Δ m = 0.1L
L = 30 000 ÷ 0.1 = 300 000J/Kg
Specific latent heat of Vaporization – Energy required to vaporize 1 kg of liquid at boiling point, with no change in temperature
The specific latent heat of steam can be calculated using this procedure:
- Part fill a beaker with boiling water and place on a balance
- Place a 50W heater in the water
- Switch the heater and wait for water to boil
- Once water is boiling start the timer and take the balance reading
- When the mass reading has decreased by 0.1 kg, stop the timer
Assume it took 4600 seconds to reduce the mass by 0.1Kg
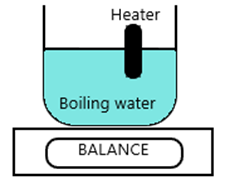
Calculations:
- Energy supplied (E) = power X time = 50 X 4600 = 230 000
- Δ m = 0.1L
- L = 230 000 / 0.1 = 2 300 000 J/Kg
The SI unit of temperature is actually Kelvin and not our usual degree Celsius/ centigrade. To convert from degrees Celsius to kelvin, we use the following equation:
T (in K) = θ (in °C) + 273
The lowest temperature that can possibly be reached is -273 degrees Celsius which is 0 Kelvin. This is referred to as ABSOLUTE ZERO as temperature in kelvin will never have a negative value. At this temperature, the particles have the LEAST kinetic energy and move the slowest.
