Simple Kinetic Molecular Model of Matter
States of Matter
Matter is any substance that occupies physical space. The kinetic theory of matter sates that matter is made of tiny particles i.e. atoms/molecules that are always in constant motion.
There are three states of matter that you need to be aware of:
There are three states of matter that you need to be aware of: Solids, liquids, and gases.
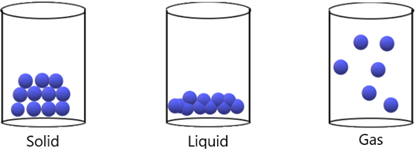
Solids have particles that are close together with strong bonds between them. It is difficult to separate the particles and as a result, solids are difficult to break apart. As particles are tightly packed, they cannot be pushed closer together so therefore solids are considered incompressible.
Liquids have particles that are close together with weaker bonds between them. Compared to a solid, the particles can move past one another and therefore liquids can ‘flow’ and be poured. The particles are still very close together and cannot be pushed before, and therefore still incompressible.
Gases have particles that are very far apart with (basically) zero forces of attraction between them. The particles can move past one another very easily. Furthermore, the particles can easily be pushed closer together and gases are thus compressible.
Temperature
Heating a substance causes the particles inside the substance to gain more energy. Particles that have more energy more quickly (i.e. more kinetic energy).
Temperature is simply the average kinetic energy of the particles within a substance. This is why heating a substance makes the temperature go up, simply because the average kinetic energy of the particles inside that substance goes up as we heat it.
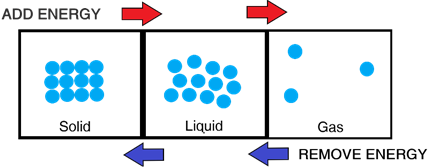
As you heat a substance, you can change the state of matter of a substance from a solid to a liquid to a gas by giving the particles more and more energy.
- In a solid, particles lack the energy to overcome the attractive forces between the particles. Hence, they are ‘stuck together’
- In a liquid, particles have more energy allowing them to gain some separation to overcome the attractive forces. Hence, they can ‘slide past’ one another
- In a gas, particles have so much energy that they completely overcome the attractive forces. Hence, they can move extremely freely.
Brownian Motion
One day, a scientist was observing a pollen grain suspended in water. He realized that the pollen grain was actually constantly moving in random directions. It looked like this:
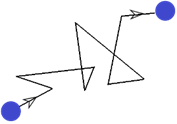
This made the scientist wonder why the pollen grain was moving in this fashion… And he figured it out.
Remember the kinetic theory of matter? Particles are always in constant motion. This meant that the water molecules were actually bombarding the pollen grain in random directions to cause the sort of movement observed above.
Brownian motion is therefore defined as the erratic random movement of microscopic particles in a fluid (i.e. pollen), as a result of continuous bombardment from molecules of the fluid (i.e. water molecules).
Pressure of a Gas
The pressure of a gas is defined as change of momentum of the particles striking the walls of the container, exerting a force.
Imagine the path taken by the two particles below (purple & green). The gas particles are always moving in a straight, but random direction. As they do so, they bounce off the walls of the container and therefore exert a force onto it.
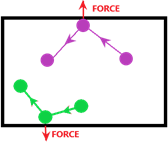
Therefore, you can see how pressure is the force exerted by the gas molecules as they strike the walls of its container.
The pressure of a gas can be altered by the temperature and the volume of the container
- A higher temperature at a constant volume
- If the size of the container doesn’t change, yet the temperature is higher, then molecules will have more energy and therefore collide with the walls more frequently and thus increase the pressure (vice versa)
- A larger volume at constant temperature
- If the temperature doesn’t change, yet the volume of the container becomes larger, then the molecules will collide with the walls less frequently and thus reduce the pressure (vice versa)
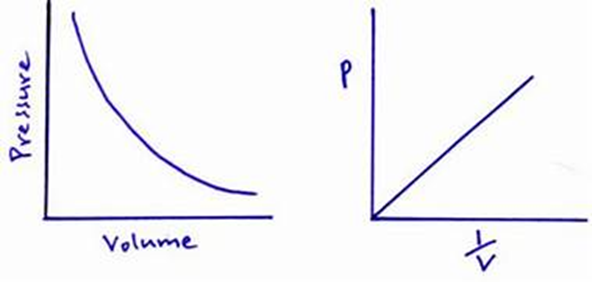
Boyle’s law states that for a fixed mass of gas at a constant temperature, the volume is inversely proportional to the applied pressure. This means that if pressure increases the volume decreases, and if pressure decrease the volume increases.
ρV = constant
[ρ = pressure, v = volume]
Evaporation
Evaporation is the change of state from a liquid to gas below the boiling point of the liquid.
All liquid particles have kinetic energy and are always moving past one another. Not all particles have the same amount of energy, some are moving quicker, some are moving slower.
Particles with a large amount of kinetic energy may overcome the force of attraction between them and other particles, and when this happens, they escape from the surface of the liquid to form a gas.
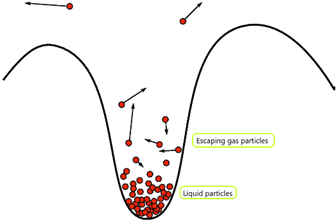
Evaporation leads to the cooling of a liquid. What we call ‘temperature’ is really just the average amount of energy the particles within a body has.
Factors that can increase the rate of evaporation would be:
- Increasing the temperature
- Increasing the surface area
- Increasing the air flow over the liquid surface
Since the most energetic particles are the ones that escape during evaporation, that lowers the overall average kinetic energy of the liquid particles, and therefore lowering the temperature.
Humans sweat when we get too hot, the sweat evaporates from the skin surface and ultimately cools the body down.
pV= constant GRAPHS: