
Radioactivity
Radioactive Decay
Radioactive is a spontaneous transformation of an unstable atomic nucleus, in which radiation is released in the form of alpha particles, beta particles, and gamma rays.
Alpha decay – In alpha decay, alpha particles are emitted from the original nucleus.
Each alpha particle is basically a helium nucleus – it has 2 protons & 2 neutrons (i.e. Z = 2, A = 4).
Therefore, in alpha decay, the proton number decreases by 2 and the nucleon number decreases by 4.
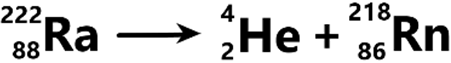
Beta decay – In beta decay, a neutron is converted into a proton and an electron. The electron is then fired out of the nucleus whilst the proton remains.
This means that the neutron number decreases by 1, and the proton number increases by 1 (therefore the nucleon number remains unchanged)
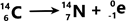
Gamma decay – In gamma decay, the number of protons and neutrons remain unchanged. The gamma ray takes away some of the excess energy of the nucleus after it has emitted an alpha or beta particle.

Detection of Radioactivity
A radioisotope is an isotope of an element that has an unstable nucleus, and can undergo radioactive decay by emitting alpha, beta and gamma radiation (or a combination of the three)
Ionizing radiation can be detected by using a Geiger-Mueller tube or a photographic film.
G-M tube – Works by detecting the ions produced when alpha, beta or gamma radiation enters the tube. It is attached to a counter that registers a count each time a radioactive particle is detected.
Photographic film – Blackened by the presence of ionizing radiation. The higher the number of radioactive particles incident on the film, the blacker it becomes.
Characteristics of Three Kinds of Emission
As mentioned above radioactive decay results in the emission of three types of radiation alpha (α), beta(β), and gamma (γ).
These are basically ‘bits and pieces’ that fall out once an unstable atom breaks apart.
All three emissions are ionizing radiations. This means that they have the ability to remove electrons from atoms that they come across. This is known as the ionizing effect.
Once an atom loses an electron, there is a charge imbalance i.e. more protons than electrons, and therefore causes the originally neutron atom to become a positively charged ion instead.
The properties of the 3 emissions is summed up in the table below:
| Alpha (α) | Beta (β) | Gamma (γ) | |
| Identity | Helium Nucleus (2p + 2n) | Fast Moving Electron | Electromagnetic Wave |
| Relative Charge | +2 | -1 | 0 |
| Relative Mass | 4 | 1÷1800 | 0 |
| Ionizing Effect | Strong | Weak | Very Weak |
| Penetration | Weak Penetration. Absorbed by a few cm of air or few sheets of paper | Greater Penetration. Absorbed by a few mm of aluminium metal. | Strong Penetration. Even lead and thick concrete cannot fully absorb this |
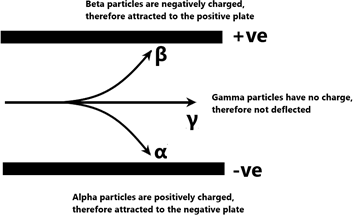
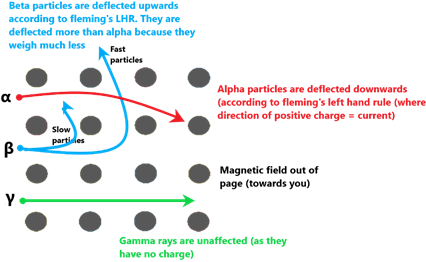
| Behaviour in electric and magnetic fields | Deflected by electric and magnetic fields | Deflected by electric and magnetic fields but to a greater extent and in the opposite direction from alpha emissions. | Not deflected by electric or magnetic fields. |
Effect of electric fields
Effect of magnetic fields
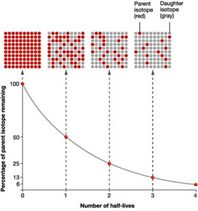
Half-life
As a sample of radioactive material decays, the activity decreases with time. The activity is the number of radioactive particles that are emitted per second.
As the number of unstable nuclei decreases, the number of radioactive particles releases per second also decreases.
It is difficult to assess when a sample of radioactive material completely halts because the activity never really falls to zero, but we can measure the half life instead.
The half-life of a radioactive isotope is the time taken for half of the nuclei in the sample to decay, or the time taken for the activity of the sample to fall to half of its original value.
Safety Precautions
Ionizing radiation can kill or damage cells by causing changes in the DNA which can lead to mutation and cancer.
It is therefore important for people working with radiation to take safety precautions. Radiation workers wear film badges, which monitor the dose of radiation received to ensure that it does not exceed the safe levels.
