Particulate Nature of Matter
Properties of solid, liquid, and gases
| SOLID | LIQUID | GASSES |
| Fixed volume | Fixed volume | No definite volume |
| Fixed shape | No definite shape | No definite shape |
| Cannot be compressed | Only compressed a little | Can be compressed |
| SOLID | LIQUID | GASSES |
| Particles close together | Particles close together | Particles far apart |
| Particles arranged in regular pattern | Particles arranged randomly | Particles arranged randomly |
| Particles vibrate around a fixed point | Particles slide over each other randomly | Particles move randomly and rapidly |
Changes of state
Solid to liquid: melting
Liquid to gases: evaporation
Liquid to solid: freezing
Gas to liquid: condensing
Solid to gas: sublimation
Gas to solid: sublimation
Note: sublimation is often asked in mcq questions
There is no temperature change while a change of state is occurring
Kinetic Particle theory
Kinetic theory: Particles behave as hard spheres, constantly moving from place to place (in liquids and gases) and vibrate upon a fixed position (in solids)
• In gases, particles are constantly colliding and changing directions resulting in random movement.
• As temperature increases, particles gain kinetic energy and move faster
• Gases can be compressed easily. Pressure and volume are inversely proportional. This means an increase in pressure at constant temperature results in a decrease in volume.
• Liquids and solids cannot be compressed easily as particles are close together and kinetic theory assumes particles are hard
• Gas in a closed container increases in pressure when it’s heated because the increased kinetic energy moves faster and collide with the walls of the container with greater force
Explanation of changes of state
• Input of energy is needed to melt and boil a substance
• Energy lessens the force of energy when a substance freezes or condenses
• Energy given out when substance freezes or condenses
• During freezing or condensation particles move slower, losing kinetic energy
Heating and Cooling Curves
Brownian motion: the random movement of particles in fluids. This happens because they are bombarded by other moving particles in the fluid.
Examples: Random movement of dust particles in still air
Diffusion: spreading movement of one substance into another due to random motion of the particles.
• Overall movement is from higher concentration to lower concentration
• Diffusion is faster in gases as particles move faster.
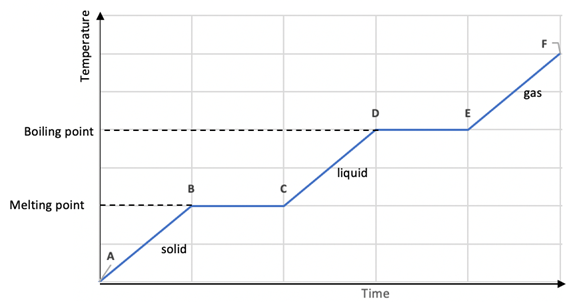
During change of state, temperature is constant.
- Evidence; Pollen grain were being moved by individual water molecules
- This is how particles are diffused
