Atoms, Elements, and Compounds
Relative Atomic Mass
Elements are made of tiny particles called atoms. These atoms are made of subatomic particles called protons, neutrons and electrons. The size is so tiny that we can’t really compare the masses using grams so we use a unit called Relative Atomic Mass (which has no units).
One Relative Atomic Mass unit is equal to the mass of a carbon-12 atom, all other elements are measured relative to the mass of a carbon-12 atom.
Ex; Hydrogen has a Relative atomic mass of 1. This means that 12 atoms of hydrogen would have the exact same mass as 1 atom of carbon
| PARTICLE | RELATIVE MASS | CHARGE |
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 1/1840 | -1 |
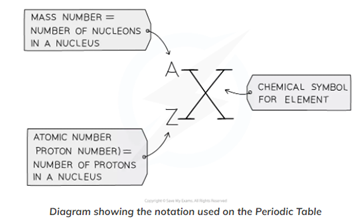
Proton Number (Atomic Number); The number of protons in the nucleus of an atom (also the number of electrons and determines the position of the element on the Periodic Table)
Nucleon Number (Mass Number); The total number of protons and neutrons in the nucleus of an atom
- Nucleon Number – Proton Number = Number of Neutrons
- Protons and Neutrons are collectively called Nucleons
- Nucleon Number is always BIGGER
Electrons
- Particles that move fast around the nucleus in paths called Shells.
- There mass is negligible, therefore the mass of an atom is contained in the nucleus
Periodic Table
- Vertical Columns; Groups= Elements in the same groups, have the same number of election in the outer shell
- Rows; Periods= Number of Electron Shells
Isotopes
- Atoms of the same element which have the same number of protons and electrons but a different number of neutrons
- It is represented by the chemical symbol followed by a dash and then the nucleon number
- There are two types of isotopes;
- Radioactive; Unstable due to the imbalance of neutrons and protons. This causes the nucleus to decay through nuclear fission and emit radiation. Decay occurs at different rates for each isotope, but the time taken for the radioactivity of an isotope to decrease by 50% is constant for that isotope and is known as the half-life.
- Medical Use; Cobalt-60 Isotope is frequently used to treat Cancer. Because gamma rays in radiation kill cancer cell more readily than healthy cells, these gamma rays are aimed at the cancer and are able to cure it
- Industrial Use; Radioactive Dating uses carbon-14 isotope to date carbon-containing materials such as organic matter, rocks and other artefacts. This is efficient as the half-life of carbon-14 is 5730 years and so it can be used to date very old objects.
- Non-Radioactive; Stable which differ only in their mass
- Radioactive; Unstable due to the imbalance of neutrons and protons. This causes the nucleus to decay through nuclear fission and emit radiation. Decay occurs at different rates for each isotope, but the time taken for the radioactivity of an isotope to decrease by 50% is constant for that isotope and is known as the half-life.
- Isotopes have the same chemical characteristics because they have the same number of electrons in their outer shells.
Electron Shell Structure
- Electrons orbit the nucleus in shells that have energy. The further away the more energy the shell has.
- First Shell; 2 Electrons
- Second Shell; 8 Electrons
- Third Shell; 8 Electrons
- The outermost shell is called Valence And an atom is more stable if it can fill this shell with electrons.
- We can represent the structure of the atom in two different ways;
- Electron Shell Diagram; Draw a diagram with a circle in the center and then shells around with the N. of electrons
- Electronic Configuration; Using Numbers and commas
- Ex; Chlorine; 2,8,7
Noble Gases
- These are the atoms in Group 8/0 and all have the outermost shell full of electrons
- This makes them unreactive and very stable
Elements
- A substance made of atoms that all contain the same number of protons and cannot be split into anything simpler
- There is a limited number of elements and they are found on the periodic table
Compound
- A pure substance made up of two or more elements chemically combined
- There is an unlimited number of compounds
- Compounds cannot be separated into their elements by physical means
Mixture
- A combination of two or more substances that are not chemically combined
- Mixtures can be separated by physical means
Metals and Non-Metals
- Metalloids are elements that have the properties of both metals and nonmetals
- Metals;
- High melting and boiling points
- Many strong metallic bonds and a lot of heat is required to break them
- Conduct Electricity
- There are free electrons available to move and carry charge
- Electrons entering one end of the metal cause a delocalised electron to displace in itself from the other end. Hence electricity is conducted
- Malleable and Ductile
- Layers of ions can slide over and take different positions
- Metaling bonds are not broken as valence electrons don’t belong to any metal
- Thus they are flexible; bent into different shaped without breaking
- Tend to be lustrous
- High density
- Form basic Oxides
- High melting and boiling points
- Non Metals;
- Do not conduct heat and electricity
- Are brittle and delicate
- Tend to be dull and non reflective
- Low melting and boiling points
- Low density
- Form acidic Oxides
Alloys
- Are mixtures of a metal with other elements (not chemically combined)
- Alloys often have properties different to the metals they contain
- Alloys contain atoms of different sizes which distorts the regular arrangement of atoms
- This makes them usually harder than pure metals
Ions
- It’s an electrically charged atom or group of atoms formed by the loss or gain of electrons. This loss or gain happens to gain a full outer shell of electrons. They will have the same structure as the one of a noble gas.
- All metals lose electrons (Become +)
- All non metals gain electrons (Become -)
- When you lose one electron you charge becomes +1 (because from 0 you have -1 negative particle)
- When you gain one electron your charge becomes -1 (because from 0 you have +1 negative particle)

Ionic Bonding
- Ionic Bonding happens because during the loss and gain of electrons, positive and negative ions are formed that attract each other. This force is called electrostatic force of attraction and forms ionic bonds
Lattice Structure of Ionic Compounds
- This refers to the arrangement of atoms of a substance 3D space
- In lattice structures, atoms are arranged in an ordered and repeating way. This is a regular arrangement of alternating positive and negative ions.
Covalent Compounds
- They are formed when electrons are shared between atoms
- Happens only with non-metal elements
- Aim is to gain a full outer shell of electrons
- 1 line between elements mean; 1 pair of electrons shared
- 2 lines between elements mean; 2 pairs of electrons shared
- 3 lines between elements mean; 3 pairs of electrons shared
Difference between Ionic and Covalent Compounds
| High Melting and Boiling Point Because the oppositely charged ions in the lattice structure are attracted by strong electrostatic force. Large amounts of energy are needed to overcome these. | Low melting and boiling point Because although there are strong bonds between the atoms in each molecule, there are weak intermolecular forces between individual molecules. Low energy is needed to overcome this forces |
| Solid at room temperature | Liquid or gases at room temperature |
| Not volatile (don’t evaporate easily) | Usually volatile |
| Water-soluble because they are polar | Not water-soluble because they are nonpolar (dissolve in organic solvents) |
| Conduct electricity in molten state or in a solution | Cannot conduct electricity |
Giant Covalent Structures
- Allotroped have giant covalent structures
- This class of substances contain a lot of non-metal atoms joined by covalent bonds
- Atoms of two different elements in a giant covalent structure
- Diamond;
- Each carbon atom bonds with four other carbon atoms forming a tetrahedron
- Strong intermolecular forces
- Does not conduct electricity
- High melting point
- Hard and dense
- Usually used in jewelry and cutting tools
- Graphite
- Each carbon atoms bonds to three other carbon atoms forming layers of hexagonal-shaped form leaving only one free carbon atom
- These free electrons exist between the layers and are free to move and carry change
- Conducts electricity (because of what stated above)
- Covalent bonds are really strong by the layers are connected by weak intermolecular forces, hence they can slide other each other making graphite slippery and smooth
- High melting point
- Slippery and soft
- Usually used in pencils and as lubricant
- Silicon(IV) Oxide
- Silicon Oxide SiO2
- Each oxygen atom forms covalent bonds with 2 silicon atoms and each silicon atoms forms covalent bonds with 4 oxygen atoms forming a tetrahedron
- Similar to diamond because of the following;
- Strong intermolecular force
- High boiling point
- Does not conduct electricity
- Hard
- Insoluble in water
- Used to make sandpaper and to line the inside of furnaces
- Diamond;
Metallic Bonding
- Metals are held together by metallic bonding
- Forms metal lattice becoming positively charged
- The valence electron no longer belongs to any metal and it is said to be delocalised
- They move freely like a sea of electrons
- They are strong bonds
- Structured with Positive ions and free electrons
