The Chemicals of life
Biological Molecules
Carbohydrates
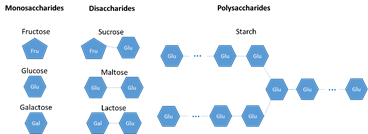
Carbohydrates are made of carbon, hydrogen, and oxygen. They are soluble in water. They are used as a source of energy for the body. One gram of carbohydrates releases 17kJ of energy. There are three types of carbohydrates: Monosaccharides, disaccharides, and polysaccharides.
- Monosaccharides (i.e. glucose) are the simplest form of sugars. They are a single unit and they cannot be broken down any further to make a simpler sugar.
- Disaccharides are two monosaccharides joined together.
- Polysaccharides are large chains of monosaccharides joined together
- Starch is a polysaccharide made of large chains of glucose and glycogen
- Cellulose is a polysaccharide made of large chains of glucose
Carbohydrates are mainly used as a source of energy.
Fats/Oils
Fats are also called lipids and are made of carbon, hydrogen, and oxygen. The oxygen content is lower than in carbohydrates. They are insoluble in water. Fats that are liquid at room temperature are called oils.
Fats have various purposes in the body:
- Source of energy. A gram releases 39kJ of energy but most cells use carbohydrates first
- Heat insulation by getting stored in adipose tissue (Cells in mammals, particularly ones underneath the skin, that become filled with large drops of fats or oils and then these stores can be used to release energy when needed)
- Myelin sheath formation
- Cell membrane formation
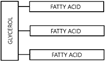
Fats are made up of three fatty acid units attached to a single unit of glycerol:
Proteins
Proteins are made of long chains of smaller molecules, called amino acids which are made of carbon, hydrogen, oxygen, nitrogen, and sometimes sulphur or phosphorus, joined end to end in a precise manner. Some proteins are soluble and some are insoluble. They are extremely important to the body and serve many different functions. here are a few:
- Growth
- Tissue repair
- Antibody formation
- Enzymes
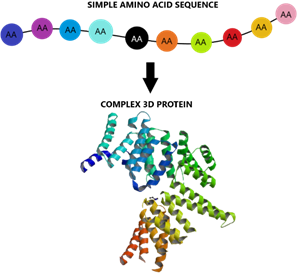
For example, each of the different colored circles represents a different amino acid. They are joined in a specific sequence as shown below.
It is really important to understand here that the final 3D structure of a protein is derived from the specific interactions between amino acids that are joined in the protein chain.
The sequence of amino acids in the chain therefore creates the final shape of the protein, and the shape is what gives the protein its function.
The diagram below represents how a single chain of amino acids eventually turns into a complex 3D protein structure with a specific function.
For example: Consider amino acids A B C D and E. The hyphens represent a chemical bond between the amino acids.
Protein 1: A-B-C-D-E
Protein 2: A-C-B-D-E
In the example above, protein 1 has amino acids A through E joined in order. Protein 2 on the other hand, has a slightly different amino acid sequence. Just from this slight difference in amino acid order, protein 2 will be completely different from protein 1 in terms of its function and structure.
Food Testing
We can test for starch, monosaccharides, proteins, and fats in a given sample via the following tests:
- Starch test [Starch test]
- Add a few drops of iodine solution
- Blue/black coloration means starch is present
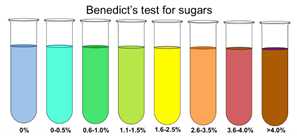
- Benedict’s test [Monosaccharide test]
- Add an equal amount of benedict’s solution into a solution of food and boil gently
- A color change (from blue) signifies the presence and quantity of monosaccharides
- Biuret test [Protein test]
- Add an equal amount of sodium hydroxide to a solution of food and mix
- Add a few drops of 1% copper sulphate
- A violet colour signifies the presence of protein
- Emulsion test [Fat test]
- Dissolve food in ethanol and pour the solution into a clean tube of water
- White emulsion signifies the presence of fat
- DCPIP test [Vitamin C]
- Add a few drops of DCPIP to a solution of food and mix
- High concentration of Vitamin C solution will take a few drops to change the colour from blue to colourless.
DNA Structure
As you may already know, genetic information is stored inside our DNA.
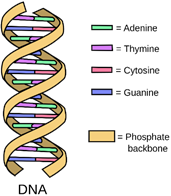
A DNA has a double helix structure whereby two strands are coiled together. Each strand has chemicals called bases. The double helix is held together via pairs of bases that are attracted to each other from one strand to the other.
Bases will always pair up in the same way. Adenine (A) will always pair with Thymine (T). Cytosine (C) will always pair with Guanine (G). The diagram above demonstrates this pairing (i.e. green is always bonded to purple and pink is always bonded to blue).
Water
Water is essential to the human body for many things. One of these things being the fact that water is an important solvent. This means that nutrients and wastes can be dissolved in water so that it can be transported around the body. Moreover, the majority of the chemical reactions inside our bodies are controlled by enzymes. Enzymes cannot work unless it is in solution (i.e. in the presence of water). Metabolic reactions in general usually require the chemicals reacting to be dissolved. Water is also needed to dissolve enzymes and nutrients in the alimentary canal so that digestion can take place. We also need water to help us to get rid of waste products and urea is dissolved in water, forming urine.
