Movement In and Out of Cells
Diffusion
Diffusion is the net movement of particles from a region of their higher concentration to a region of their lower concentration down a concentration gradient, as a result of their random movement.
The constant random movement of particles (and their kinetic energy) allows diffusion to occur. Ultimately this means that particles will spread out.
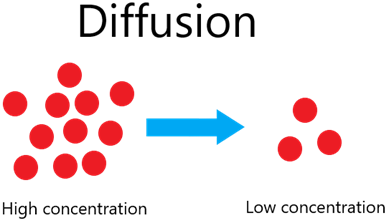
It is also important to understand that diffusion is quite often how molecules move in and out of our cells through the cell membrane.
For example, the diagram below demonstrates a cell surrounded by nutrients (red dots). We can see that on the left, there are a lot more nutrients outside the cell than inside the cell. By diffusion, the nutrients will diffuse into the cell (from higher to lower concentration) until the number of nutrients inside and outside the cell is balanced
There are certain factors that affect the rate of diffusion:
- Surface area: The larger the surface area, the higher the rate of diffusion. This is because more molecules at a given time will be diffusing.
- Temperature: The higher the temperature, the higher the rate of diffusion. This is because molecules are faster and have more kinetic energy with higher temperatures.
- Concentration gradients: The higher the concentration gradient, the higher the rate of diffusion.
- Distance: The shorter the distance, the higher the rate of diffusion. This is quite self-explanatory. The shorter the distance the particles have to move, the quicker the process is going to be.
Osmosis
Osmosis is the net movement of water molecules from a region of high-water potential (dilute solution) to a region of low-water potential (concentrated solution) through a partially permeable membrane.
Think of osmosis as the diffusion of water across a partially permeable membrane. When we are talking about water, we cannot use the term ‘concentration’ anymore because a concentration denotes the amount of substance dissolved in water.
Because water cannot be dissolved in water, we need to use another term instead: Water potential.
- For a very dilute solution, because it has a lot of water, it has a high-water potential.
For a very concentrated solution, because it has less water, it has a low water potential
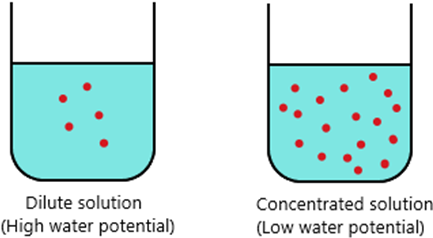
So, let’s apply this concept to osmosis. Refer to this diagram:
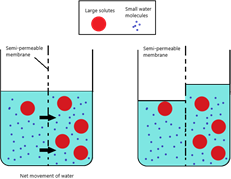
The left-hand side of the beaker has fewer solutes dissolved so therefore, the solution is more dilute (or less concentrated) compared to the right-hand side.
Under normal circumstances, the sugar molecules themselves will diffuse across from RHS to the LHS via diffusion (as we talked about earlier). However, the sugar molecules are too large to pass through the partially permeable membrane (e.g.: visking tubing), and therefore cannot diffuse.
Water molecules, however, can pass by freely through the membrane. The molecules will travel from the region of high-water potential to low water potential so therefore, in this case, water will move from the RHS to the LHS via osmosis.
Cell membranes are partially permeable so cells absorb or remove water via osmosis.
Consider these scenarios:
- Adding cell into pure water / dilute solution
- There will be higher water potential outside the cell than inside the cell and therefore water will move into the cell
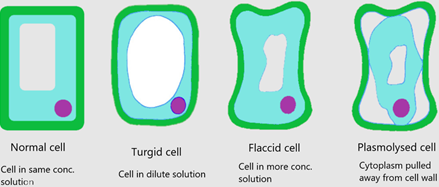
- As water enters the cells they become ‘turgid’
- An animal cell can burst if too much water enters
- A plant cell has support from its cell wall and therefore will most likely maintain its turgidity without bursting
- Adding cell into a concentrated solution
- There will be higher water potential inside the cell than the outside and therefore water will move out of the cell
- As the water moves out, cells become ‘flaccid’
- An animal cell can become crenated if too much water is lost
- A plant cell can become plasmolyzed if too much water is lost. This is when the cytoplasm shrinks due to the loss of water but the cell wall fails to shrink due to its tough structure. The cytoplasm eventually tears away from the cell wall.
Active Transport
Active transport is the movement of particles through a partially permeable membrane from a region of lower concentration to a region of higher concentration using energy from respiration.
Active transport is used in cases where diffusion or osmosis cannot be relied upon. For example, what if a cell wanted to absorb extra nutrients from outside the cell despite having a higher concentration of those nutrients inside the cell? Diffusion wouldn’t work because the concentration gradient is going the opposite way. These situations are encountered frequently in:
- Plant root hairs (molecules from the soil are taken in against the conc. gradient)
- Villi epithelial cells (glucose from the lumen of the intestine is taken in)
Active transport uses energy to oppose the concentration gradient and forcefully transport molecules against it. Here is a simple diagram to help you visualize how it all works:
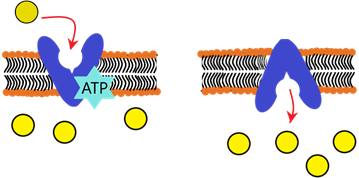
In the cell membranes of all cells, there are certain embedded protein molecules that carry out this process. The protein basically ‘captures’ the molecules from one side of the cell, and it changes shape in a way to transport the captured molecules to the other side of the cell. Energy (from respiration) is required to alter the protein shape (referred to as ATP – Adenosine triphosphate).
