Enzymes
A catalyst is a substance that increases the rate of chemical reaction and is not changed by the reaction itself.
An enzyme is a biological catalyst that catalyzes many important reactions inside an organism (such as respiration) and therefore necessary to sustain life. All enzymes are proteins and are named after the substance they break down. How enzymes work is more easily shown through a diagram. Take a look below:
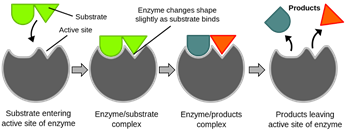
Firstly, a substrate is a substance that enzymes act upon. It is important to understand that enzymes are very specific, and the reason for their specificity lies in their active sites – a region of an enzyme that binds to a particular substrate. The shape of the active site of an enzyme is complementary to only one specific substrate (lock and key mechanism).
As demonstrated in the diagram, the green substrate has a shape that pairs impeccably with the shape enzyme’s active site. As the enzyme binds with the substrate, an enzyme-substrate complex is formed. The reaction then occurs on the enzyme and the enzyme-product complex is formed. The products eventually leave the enzyme.
Enzyme Activity
There are certain factors that can impact enzyme activity. All enzymes have an optimum temperature and an optimum pH. These are certain temperatures or pH in which a particular enzyme works best in, and it can vary between different enzymes.
For any given enzyme, if the conditions stray too far from the optimum, then denaturation can occur. This is when extreme non-ideal conditions (i.e. high temperatures or very low/high pH) causes chemical bonds in the enzymes to break apart. This results in the change in shape of the enzyme’s active site. Remember, the active site has a very special shape and it fits only one specific type (and shape) of substrate. An alteration in active site will therefore cause the enzyme to lose function.
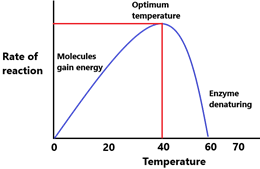
Temperature v/s Enzyme Activity – Low temperatures reduce the rate of chemical reactions in general. This is because molecules need to collide with one another and have enough energy for a reaction to occur. In low temperatures, molecules are traveling at lower speeds (less energy) and therefore the rate of successful collisions are lower. Moreover, even when collisions do occur, the molecules may have insufficient kinetic energies to begin with, and therefore the reaction may not occur. Enzyme activity is therefore low in low temperatures. It is important to note however, that low temperatures do not denature enzymes.
Higher temperatures generally increase the rate of chemical reactions. Molecules are faster and have more kinetic energy. This means that the rate of successful molecular collisions is higher, and most molecules will have sufficient energy required for the reaction. However, temperatures that are far beyond the optimum temperature of the enzymes can start to denature it, and reduce enzyme activity as a result. Most enzymes have an optimum temperature of approximately 37 degrees in the human body, and start getting denatured at above 50 degrees.
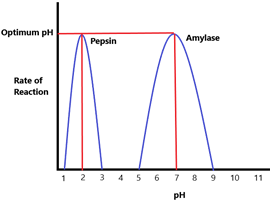
pH v/s Enzyme Activity – The optimum pH of an enzyme can vary. Pepsin is an enzyme found in the stomach’s acidic conditions and therefore made to work best in a pH of approximately 2. Amylase on the other hand, is found in saliva (more neutral conditions) and therefore has an optimum pH of 7. Very high or very low pH’s can denature these enzymes if it deviates too much from their optimum.